
Continuous environmental monitoring

Single to multi-site installation

Access data from any mobile device

Monitor any environmental parameter or sensor

Fully scalable system from one, to thousands of sensors
Pharmaceutical grade mounting system specifically designed to comply with multiple industry regulations
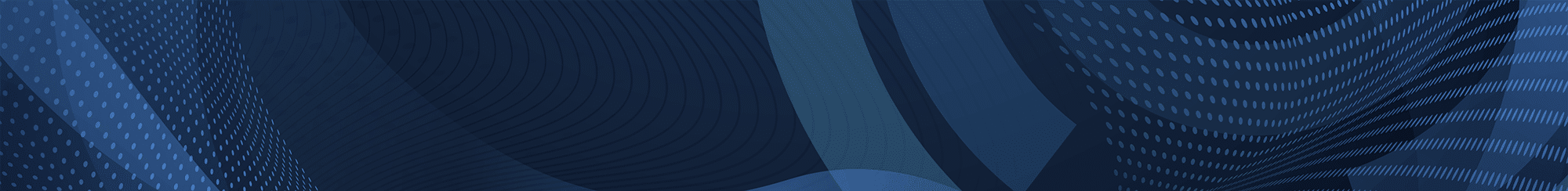
Continuous environmental monitoring of key parameters
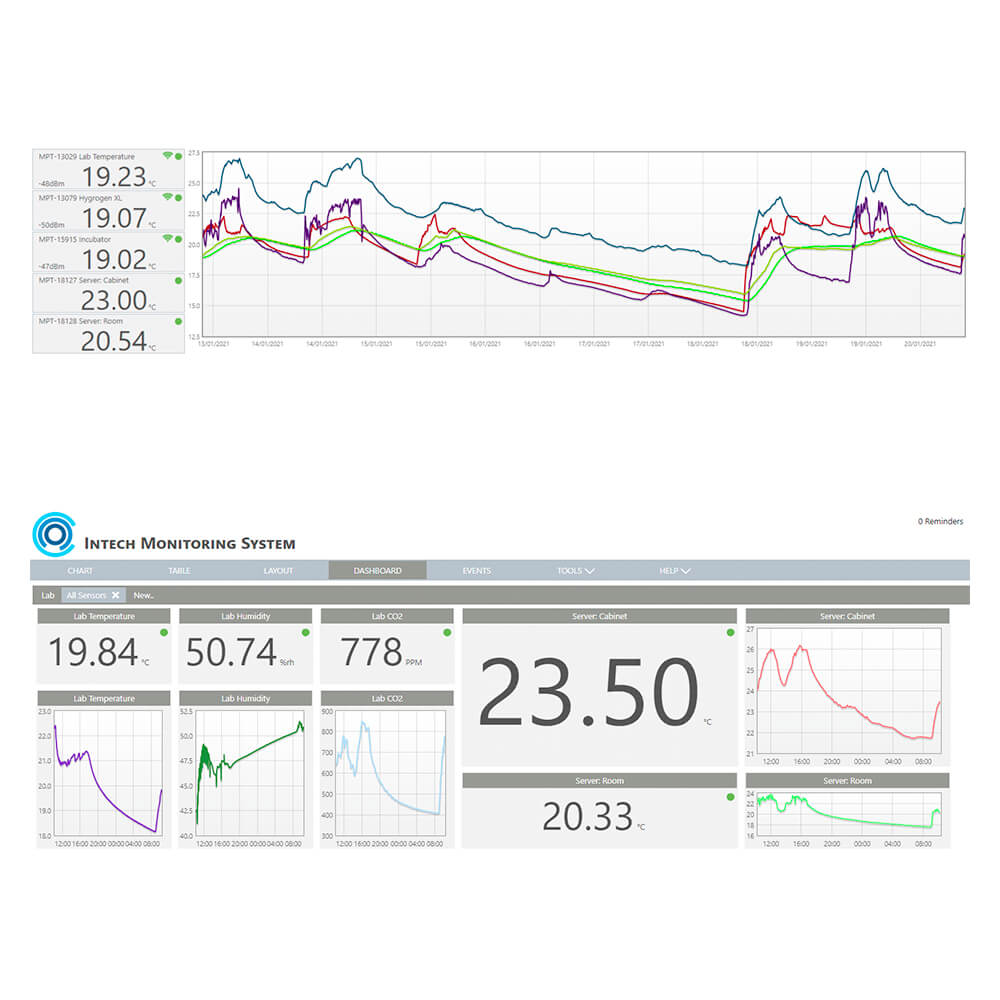
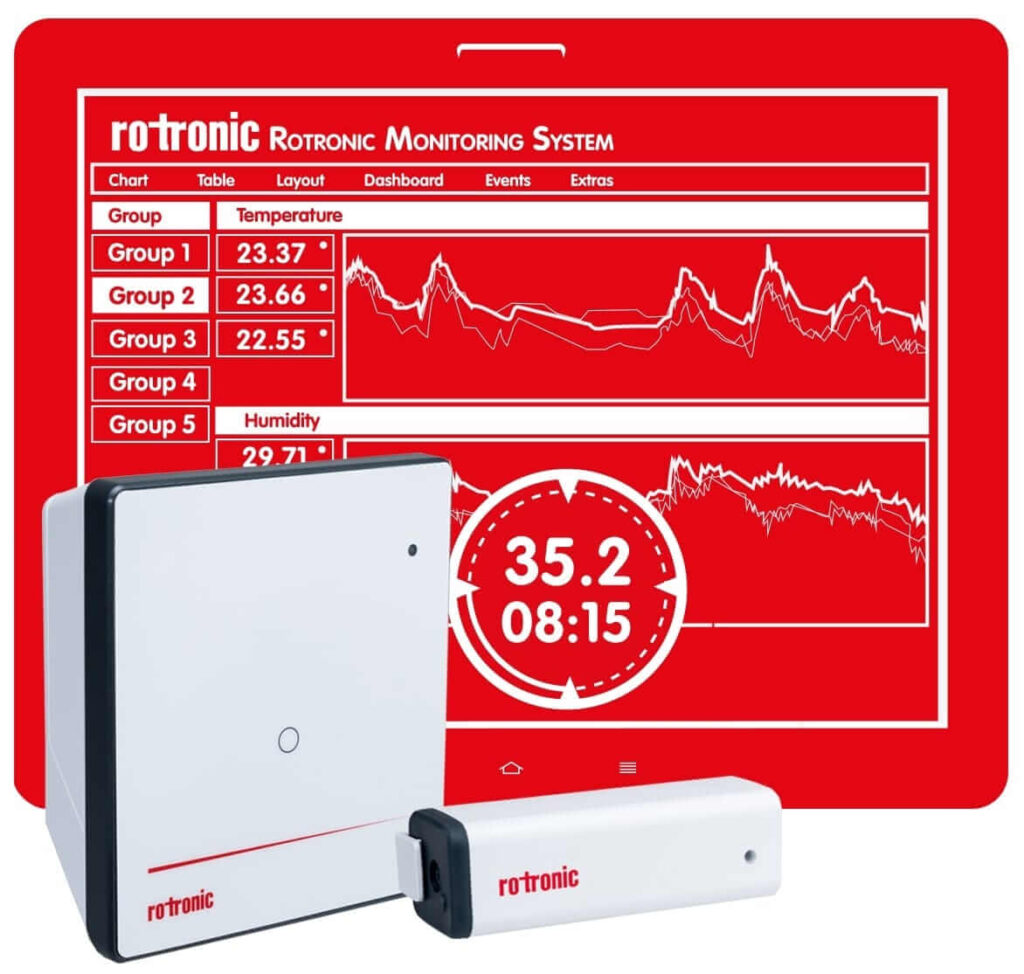
The Rotronic Monitoring System (RMS) is a modular system of hardware elements and web-based software. It provides maximum flexibility in installation and ensures readily available data. The data loggers record measurements from Rotronic and third-party sensors, and transmit them to the secure database. Information is stored and made available to users at any time around the world via PCs, Macs, tablets or smartphones.
RMS – For any application
RMS is one of the most flexible monitoring systems on the market today. From small applications with one measurement point, to larger systems with several thousand measurement points, RMS offers tailor-made solutions. Existing hardware can be integrated into the Rotronic system and, vice versa, Rotronic hardware can be incorporated into existing software according to your wishes and requirements for a continuous monitoring system.
RMS is used across a broad range of industries and has been built from the ground up to meet the stringent requirements of the pharmaceutical and healthcare sectors. RMS is regularly used to monitor the following temperature-controlled storage equipment and facilities:
- Fridges and freezers
- Incubators
- Stability chambers and environmental test chambers
- -20°C and -40°C freezers
- ULT freezers (-70°C and -80°C)
- Cryostorage facilities and equipment (LN2)
- Warehouses and product storage rooms
- And more
RMS is also used to monitor:
- Server rooms (temperature and humidity)
- Data centres (temperature and humidity)
- Cleanrooms (temperature, humidity, airborne particles, light, differential pressure etc.
- And more
FDA Conformity
RMS is fully compliant to FDA CFR 21 Part 11, electronic records; electronic signatures, as well as EU Annex 11 (EUDRALEX).
Our R&D team work on the basis of the GAMP5 recommendations and the RMS is built to meet these requirements. The RMS software is a category 4 software.
Environmental Monitoring Systems

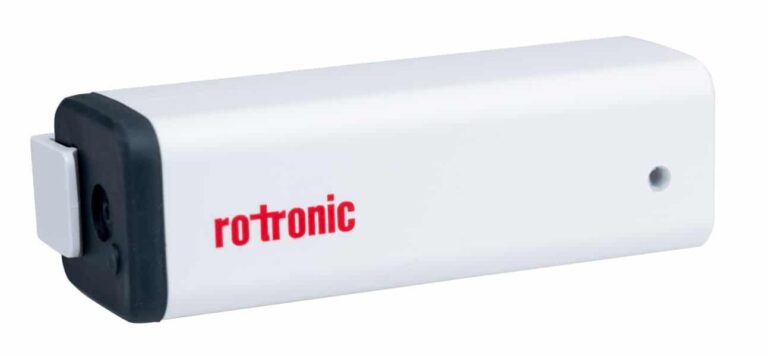
RMS-MLOG-T-868 – Wireless Mini Data Logger – Temperature
Product details
RMS-LOG-L – Data Logger – LAN Interface
Product details
HCD-S – Humidity and Temperature Probe
Product details
